
What is ammonia?
Ammonia is a combination of hydrogen and nitrogen with the chemical formula NH3. This colorless substance with a pungent odor is a nitrogen scavenger that is of interest as a precursor to food and fertilizers. This product is directly and indirectly one of the components of medicine and is used in many cleaning products. Contact our experts to buy ammonia and inquire about its selling price.
Azpog Group, with long experience in supplying all kinds of chemicals with the best brands available at the international level, is ready to cooperate with various production and industrial units.
What is ammonia?

Ammonia with the chemical formula NH3 is a colorless gas with high irritant properties and pungent smell. It has a molecular weight of 17.031 grams and a boiling point of 33.34ºC. It is a colorless substance with a pungent odor and in pure gaseous form. Ammonia is used as a precursor for food, fertilizers and chemical products.
It dissolves easily in water and forms ammonium hydroxide solution, which is highly irritating and causes burns. Ammonia gas under pressure easily condenses and turns into a colorless and transparent liquid. It can be obtained from natural and synthetic sources.
Physical and chemical characteristics:
Ammonia is used domestically and industrially. Also, as you know, this substance is in its pure form as gas, which is liquefied and used as liquid ammonia for ease of use and easier transportation. Below is the structure. You are viewing this item.
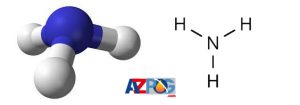
Industrial ammonia is usually sold as a 28% solution in water or under pressure and low temperature. This molecule is polar and its hydrogen bonding ability makes it dissociable in water.
Properties of ammonia
molecular formula: NH3
Molecular weight: 17.031 g/mol
Color: Colorless and pungent smell
Freezing point: -77.7 degrees Celsius
boiling point: -33.34 degrees Celsius
Solubility in water: 54 grams per liter
Ammonia production methods:
This substance is produced naturally and industrially, which we will describe in the following methods:
Natural resources:
This chemical is found in small amounts in nature, nitrogen is produced from animal and vegetable matter, and ammonium salts are found in small amounts in rainwater. While ammonium chloride is found in small amounts in volcanic areas, the kidneys secrete ammonia to neutralize excess acid, ammonium salts are released from fertile soil and distributed into seawater.
Industrial method of production:
The above product is one of the most widely used mineral chemicals. In 2018, the amount of production of this substance in various countries was as follows:

Before the start of World War I, most of the ammonia was produced by dry distillation of plant and animal waste nitrogen which was reduced by distillation of nitric acid and nitrate with hydrogen. Today, this material is also produced by distilling coal and breaking down ammonium salts.
NH4Cl + 2 CaO → CaCl2 + Ca(OH)2 + 2 NH3
On a laboratory scale, urea and calcium hydroxide can be produced from heat.
(NH2)2CO + Ca(OH)2 → CaCO3 + 2 NH3
But the main method of producing this substance is known as the Harbor method, in which hydrogen converts atmospheric nitrogen into ammonia with a metal catalyst at high temperature and pressure.

The most commonly used catalysts in this process include aluminum oxide, silicon dioxide, and beryllium and vanadium oxides.
Ammonia reactions
- This substance is decomposed into its constituent elements at high temperature and in the presence of a suitable catalyst. Its decomposition is an exothermic process that requires 5.5 kcal/mol of ammonia and produces hydrogen and nitrogen. The above product can be used as a source of hydrogen for acid fuel cells. Among the most active catalysts, rutinum and platinum can be mentioned, while nickel is not such an active catalyst.
- Ammonia is a weak base that combines with acids to form salts, for example with hydrochloric acid, ammonium chloride; With nitric acid, ammonium nitrate is made.
- Ammunia needs moisture to combine with hydrogen chloride and react. If you put an opened bottle of concentrated Ammunia and hydrochloric acid, clouds of ammonium chloride are formed according to the following reaction.
NH3 + HCl → NH4Cl
- Although the mentioned substance is a weak base, it can play the role of a weak acid and form an amide, in other words, ammonia can be considered a buffer. For example, in the reaction with lithium, lithium amide is produced.
2Li + 2NH3 → 2LiNH2 + H2
- Amines can be produced by the reaction between this substance and alkyl halides. Due to the nucleophilic nature of the NH2 group, amines of the second and third types are produced as side products.
- The burning reaction of ammonia is an exothermic reaction, although this substance does not burn easily except when it is mixed with air at a ratio of 15-25%. The flame resulting from this reaction is green
4NH3 + 3O2 → 2N2 + 6H2O (g)
ΔH°r = −1267.20 kJ
Reaction with water:
Ammunia reacts with water as follows. This substance is completely dissolved in water and heat is released as a result.
NH3 + H2O ⇌ +NH4 + −OH
applications
This substance has many industrial and domestic uses. In the past, fermented urine that formed ammonia was used to wash clothes and remove hair from the skin to prepare for tanning and prevent iron from rusting. Other uses of this material are as follows:
Ammonia in chemical fertilizer:
The main use of this substance is as a fertilizer. In 2019 in America, approximately 88% of this substance was used as fertilizer or as salt. When this substance enters the soil, it helps to increase the yield of crops such as corn and wheat. 30% of agricultural nitrogen in the United States is in the form of anhydrous ammonia, and 110 million tons are used annually in the world.
Making nitrogenous compounds:
Ammonia is used directly and indirectly in the manufacture of most compounds containing nitrogen. One of the production compounds is nitric acid, which is used to produce fertilizers, explosives, etc. Other compounds that this substance plays a role in producing are shown in the figure below:
Also, this substance is used in the production of products such as ammonium nitrate, dinitrogen tetroxide, ethanolamines, hexamethylene tetraamine and ammonium bicarbonate.
Use for washing:
Homemade ammonia is NH3 solution in water. It is used as a general cleanser for surfaces. Because it creates transparency, the most common cleanser for cleaning glass, porcelain and stainless steel is also used to clean the furnaces to remove the remaining particles. The concentration of this substance in domestic use is 5 to 10 percent. Not rewarding this product with water and its use can be associated with severe damage and skin burns.
Use in fermenters:
Ammonia solutions from 16 to 25% are used as an industrial fermenter as a source of nitrogen for microorganisms and pH adjustment during fermentation.
Antibacterial agent for food products
In 1868, it was found that the mentioned product is highly antiseptic. Currently, anhydrous ammonia is used to eliminate microbial contamination of beef.
Correction of gas emissions:
Ammonia is used to remove SO2 from fossil fuels, and the resulting product is converted to ammonium sulfate for use as fertilizer. This substance neutralizes nitrogen oxide pollutants produced by diesel engines, which this process It is called selective catalytic reduction (SCR).
Application as fuel:
The raw energy density of liquid ammonia is 11.5 MJ/L, which is one third of diesel. For this reason, the production of hydrogen from this material creates an opportunity to be used in hydrogen fuel cells or directly in high temperature fuel cells. Conversion of the above product to hydrogen through the sodium amide process is used for purposes such as combustion or for fuel in proton exchange membrane cells.
The conversion to hydrogen is about 18% compared to hydrogen gas under pressure (5%) and it will have a much lower cost than hydrogen that must be kept compressed.
Ammonia engines can work using the liquid form of Ammunia because it does not have a carbon atom, the fuel of this substance does not produce pollutants such as carbon dioxide and carbon monoxide or soot, and also because of its high octane number (120) and high flame temperature. , this material is a good option to use instead of fossil fuels.
In rocket engines, the mentioned material is also used as fuel. It may not be as powerful as other fuels, but it does not produce soot in the rocket engine, and its density is compatible with oxidizers and liquid oxygen, which simplifies the design of the aircraft.
Ammonia as a respiratory stimulant:
The vapor released from this substance has found significant use as a respiratory stimulant. Methamphetamine is an illegal substance that is produced from this substance, but in the production method of this substance, alkali metals and ammonia are placed together, which may cause an explosion due to the high temperature of this substance when the reagents are added.
Application in the production of coolers:
Due to the evaporation properties of ammonia, it is a useful material for cooling. This material has been of interest before the popularity of chlorofluorocarbons. The above product is used in dry form in industrial refrigeration applications due to its high energy efficiency and low cost.
Other uses:
Other applications of this material that have more limited uses include:
textiles:
Liquid ammonia is used to purify cotton, especially in washing wool, and it is also used to make synthetic fibers such as nylon and rayon.
Lift gas:
At standard temperature and pressure, this substance is less dense than the atmosphere and has about 47% of the lifting power of hydrogen and helium.
Wood Industry:
This product is used to darken white woods such as oak. Ammonia vapors react with the particles in the wood and do not change its color.
Dangers of ammonia:
- In contact with the eyes, it may cause side effects such as pain, redness and watering. After eye contact with this substance, wash with plenty of water.
- When it comes into contact with the skin, it causes pain, burning, redness, and sometimes burns.
- In case of inhalation, expose the poisoned person to fresh air.
- Swallowing this substance in the abdomen may cause severe pain.
The American Occupational Safety and Health Administration has considered the amount of exposure without harm in an environment with a concentration of 35 ppm as 15 minutes and the amount of exposure in an environment with a concentration of 25 ppm as 8 hours. Ammonia is a dangerous liquid because it is a humectant and causes caustic burns.
Environmental effects:
Waste generation should be minimized as much as possible, disposal of solutions and any by-products should be done in accordance with the requirements of environmental protection law.
Ammunia is toxic to aquatic animals even in low concentration and therefore dangerous for the environment. This substance can cause problems for aquatic organisms such as reduced fertility and poor growth.
Maintenance
- This material is stable and under normal storage conditions, destructive and harmful reactions do not occur.
-
It should be away from possible sources of ignition (spark or flame) and do not put containers under pressure, cutting, welding, or soldering.
-
Avoid incompatible materials such as oxidizers and yellow metals such as brass and copper
Place the material storage container vertically -
Avoid pulling, rolling or pulling the cylinders, use a hand winch for transport
-
Store in a well-ventilated place
question and answer:
In the end, we need to answer some important questions and points about this article.
Combination with bleach is prohibited!
One of the things that is always denied is the combination of cleaning products with each other, the biggest risk that you may face when using this material is the inhalation of chloramine gas caused by combining chlorine-containing bleaches with ammonia, this poisonous gas. and breathing it will cause serious damage to the respiratory tract, so if you care about your health, always remember the sentence that it is forbidden to combine with bleach.
Why is it important to measure blood ammonia?
Since this substance is also present in the blood, its level should be periodically checked and controlled through tests. The permissible amount of ammonia in the blood is equal to 15 to 45 micrograms per deciliter, and higher amounts can cause memory loss, high blood pressure, convulsions, and even coma.
Frequently Asked Questions
What is the use of ammonia?
Almost more than 80% of ammonia produced in industry is used as fertilizer in agriculture. It is also used as a refrigerant gas, water purification, plastic production, paint, household and industrial detergent solutions.
What effect does ammonia have on the human body?
Ammonia can enter the body through inhalation, ingestion or skin contact. This compound is highly corrosive and dangerous if it comes into contact with the body.
What are the effects of ammonia?
Ammonia is a highly corrosive compound. A high concentration of this substance can cause burns to the eyes, nose and throat. It can even cause death.
Ammonia sales and price inquiries:
Wholesale sales of all kinds of chemicals with the best quality and the most suitable price, just complete your purchase order by contacting our experts.



